Leading Question
A similar method for the addition of Br using LiBr in 2-heptanone with inversion of stereochemistry could be completed using PPh3 and CBr4 in CH2Cl2. Together these reagents constitute an Appel reaction, conversion of an alcohol into an alkyl halide. A large driving force is present for the forward reaction due to formation of solid triphenylphosphine oxide product. The reagents for the conversion of product 26 to 27 were replaced with those below. Instead of making the hydroxyl group a good leaving group, this step could achieve the inversion of stereochemistry directly.
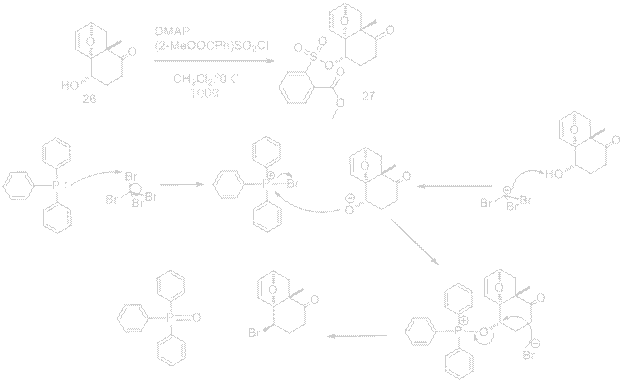

PPh3/CBr4 References:
Aoyagi, S.; Tanake, R.; Naruse, M.; Kibayashi, C. Tetrahedron Lett. 1998, 39, 4513-4516.
Chen, M.; Taylor, S.D. Tetrahedron Lett. 1999, 40, 4149-4152.
Bhattacharya, S.; Haldar, S. Proc. Indian Acad. Sci. 2002, 114, 197-201.


